Stages of Embryo Development
After an egg is retrieved and fertilized, the process of embryo development to the moment of embryo transfer or embryo biopsy is a mystery to most patients. Indeed, the biology of embryo development is complicated, but it shouldn’t be a complete black box to patients anxiously awaiting results.
At the time of oocyte retrieval, a needle is used to aspirate the developing ovarian follicles in which the oocytes (eggs) have been developing over the preceding days. The mechanics of follicle development from the start of ovarian stimulation is also a complicated topic best left for a different essay, but it is reasonable to compare the pattern of events to the normal menstrual cycle. For example, just as natural menstrual cycles will vary in length from person to person and additionally cycle to cycle, the timing of an egg retrieval procedure can vary from person to person and cycle to cycle.
The timing of the egg retrieval can be critically important to successful treatment. An analogy may be helpful: imagine that all of the fruit from an apple tree must be picked at the same time. To maximize harvest of the greatest number of ripened fruit and to minimize wastage (overripe or underripe fruit), the decision about the time to pick the fruit is very important. To extend the analogy, measurement of the sizes of the apples would be a useful to optimize the probability of making the correct decision. If all of the apples were very small, it is likely that most of the fruit is underripe; too big, and the fruit may have spoiled on the tree. Similarly, the size of the ovarian follicles is a useful estimator for when the eggs inside should be mature, to optimize the yield of mature, good quality eggs.
When the egg retrieval is performed, the embryology lab will receive the oocytes and must assess them for maturity. There are several stages of oocyte maturity variously termed and also given shorthand abbreviations such as, “GV,” “MI,” and, “MII.” These are descriptors of the biological stage of development. Suffice it to say, only MII oocytes are mature and are capable of successful fertilization. MI is the step preceding MII, and GV before MI. It is also possible to have “post-mature” oocytes that don’t function properly, like the aforementioned overripe fruit. Some MI oocytes might mature in the lab environment, but it is rare for a GV to move forward two steps after retrieval. That is, once picked, the fruit will generally not continue to ripen. Without knowing how a patient will respond to treatment, Reproductive Endocrinologists must make an educated guess about the appropriate time to, “harvest,” the oocytes. Performed too soon and there will be many immature oocytes, and too late, too many post-mature, underperforming ones. Future treatment cycles can be adapted to a patient’s personal response once some specific data about that person has become available from the experience of treatment. For most patients, though, that won’t be necessary. Notably, there are sometimes other reasons for immature oocytes rather than a retrieval performed too soon, but those rare cases are beyond the current discussion.
After retrieval, the mature oocytes are exposed to sperm in order to allow fertilization to occur. Fertilization is not simply the entry of a sperm cell into an egg cell, but rather the biological reaction that occurs after that event. The day of the egg retrieval is termed, “Day 0,” and subsequently, “Day 1,” is when the oocytes are assessed to see which have become embryos. Embryologists can assess this by examining the embryos under a microscope and by identifying cellular structures that only appear as a consequence of successful fertilization. This is not to say that the embryos are normal, because as-yet unidentifiable genetic errors may still lurk undetected. A typical fertilization rate may range from about 70-80% of the mature eggs retrieved.
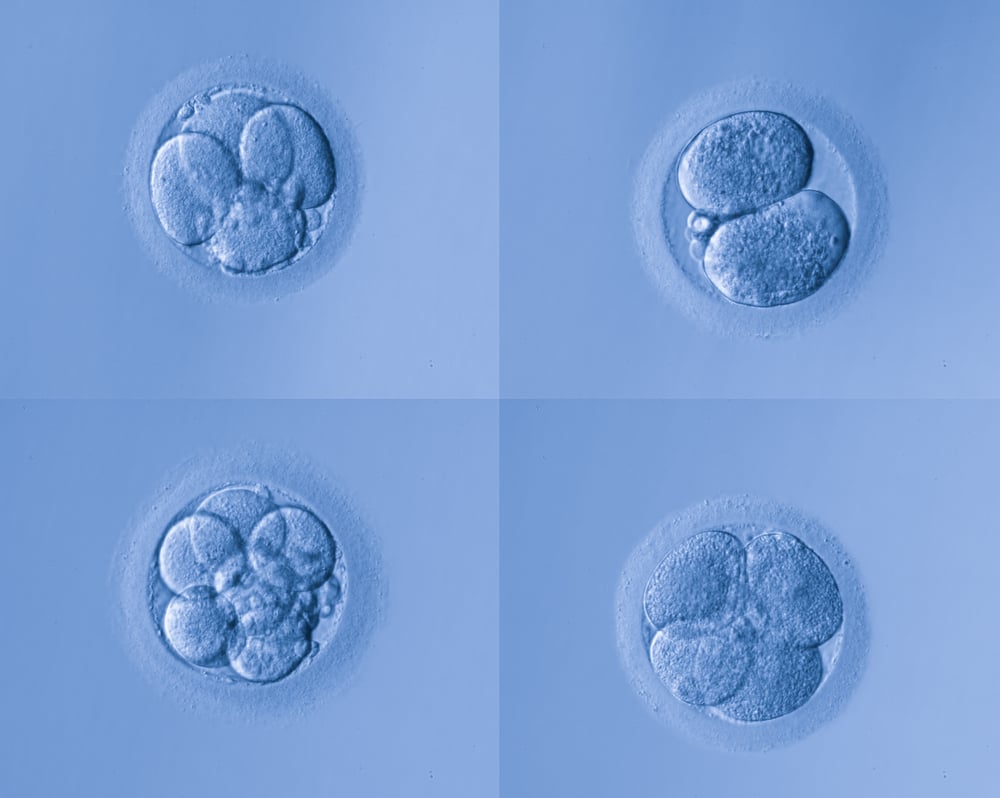
After fertilization, cell division begins. We refer to these embryos in the first few days of development as, “cleavage,” stage embryos, because we can count the number of divisions (cleavages) under a microscope. Classically, the cleavage stage lasts until “Day 3” after retrieval. Examining the number of cell divisions and other characteristics observable under the microscope can provide a way to rank one embryo as being more likely to create a pregnancy than another. Starting on Day 3, when the ideal embryo has 8 distinct cells, cellular activity becomes significantly more complex as additional genes are activated to further the development of these cells.
On Day 4, embryos enter the, “morula,” stage, a compacted mass of cells. (Morula derives from the Latin word for mulberry.) And on Day 5, the embryo re-expands, like blowing up a collapsed balloon, into a, “blastocyst,” stage embryo. Blastocyst embryos have two distinct cell groups, an inner cell mass attached to the inside of a globe of outer cells. These two cell types are now, “differentiated,” cells, whereas before they were not. Early embryonic cells are, “stem cells,” in the sense that they have not committed to becoming a specific cell type, such as muscle or hair, but that commitment is referred to as differentiation. Once differentiation is started, the cell cannot go back to being another type of cell, even though every cell with a nucleus contains within its DNA an entire blueprint for the creation of every cell type and for performing every metabolic function. In blastocyst embryos, there is an inner cell mass that can become any of the cell types of the fetus, which is surrounded by trophectoderm cells forming a globe around it. Trophectoderm cells can only become future placental cells but not future fetal cells. Likewise, the inner cell mass cells cannot reverse course to become placental cells.
It's important to understand that this timeline, from fertilization to blastocyst stage, is an idealized progression. Some embryos may develop a little slower or a little faster than this timeline. The rate of this progression also has implications for the likelihood of success: slow growing embryos are frequently less likely to implant; however, a slow growing embryo isn’t necessarily abnormal. And, due to random genetic error, some embryos will begin to grow, but then will, “arrest,” or stop growing, because they are not normal.
Blastocyst embryos can be described by their “stage,” of development. As features become more apparent and as the embryo literally expands, the stage increases, starting with stage 1. An “expanded” blastocyst is typically Stage 3 or 4, whereas one beginning to escape from its shell (yes, human eggs have shells too!), is Stage 5, and a completely hatched one is Stage 6. In the expanded stages (starting at Stage 3), the inner and outer cell groups can be seen separately when observed under a microscope, allowing embryologists to give each a grade to describe their quality. Higher quality grades are associated with higher implantation rates. In our lab, expanded blastocyst stage embryos are given a stage number and two letter grades, the first grade corresponding to the inner cell mass, and the second to the outer cells. For example, a stage 5AB blastocyst is hatching, has an inner cell mass grade A, and has outer cells that are grade B. In our lab, both cell groups are graded A-D, like in school. The second grade is not a modifier of the first, but rather a distinct grade, again like two different subjects in school, say, Mathematics and History. There are nuances of cell structure that embryologists use to assign letter grades. In terms of quality, the speed of embryo development also matters, so a Day 5 5AA is more likely to implant than a Day 7 5AA, even though they are the same stage and have the same grades. We know these differences from observing the outcomes of our patients when different embryos are used. Many embryology laboratories do not culture embryos to Day 7 and instead stop at Day 6, but we have data to support using them. Only embryos that meet minimum criteria will survive embryo cryopreservation and subsequent thaw; there are also minimum criteria to determine when an embryo can be biopsied.
Comparing cleavage stage embryos to blastocyst embryos is akin to comparing apples and oranges, as the adage goes, because they are developmentally very different. The advantages of extended embryo culture to the blastocyst stage is the subject for a different article, but suffice it to say, the more information that can be learned about an embryo, the better selection can be made to maximize the probability that a chosen embryo will result in a successful pregnancy.
